Cardiovascular Disease
The presence of type 2 diabetes is an established risk factor for cardiovascular disease (CVD), and CVD is the number one cause of death in people with diabetes.1 Patients who experience episodes of severe hypoglycemia have a six-fold increase in diabetes-related deaths.2 The risk of death is elevated in individuals who have both CVD and severe hypoglycemia episodes.3 The risk of incident heart failure in people with type 2 diabetes is twice that of people who do not have diabetes.4
Glycemic control is associated with reductions in the risk of microvascular complications; however, a 2018 report by Lee and colleagues that detailed a prospective cohort analysis of 1209 participants showed that severe hypoglycemia—at least one incident during a median follow up of 15.3 years—was associated with multiple adverse and long-term effects. After a severe episode of hypoglycemia, the three-year cumulative incidence of coronary heart disease was 10.8% with a mortality rate of 28.3%. Hypoglycemia was also associated with cancer mortality. Similar results were shown in all groups regardless of age, sex, ethnicity, duration of diabetes, and baseline risk for cardiovascular disease.5
A 2017 report from this same group of investigators evaluated nontraditional risk factors associated with severe hypoglycemia in 1206 patients with diabetes.6 Severe hypoglycemia was strongly associated with poor glycemic control, glycemic variability as evidenced by 1,5-anhydroglucitol, kidney damage, and measures of cognitive and functional impairments.6
The impact of hypoglycemia must be considered, both in terms of cardiovascular risk and other types of adverse events. Repeated episodes of hypoglycemia can impair counterregulatory hormone release and potentially lead to hypoglycemia unawareness. Short- and long-term complications of hypoglycemia include not only acute cerebrovascular disease and myocardial infarction, but also neurocognitive dysfunction, retinal cell death and loss of vision.2
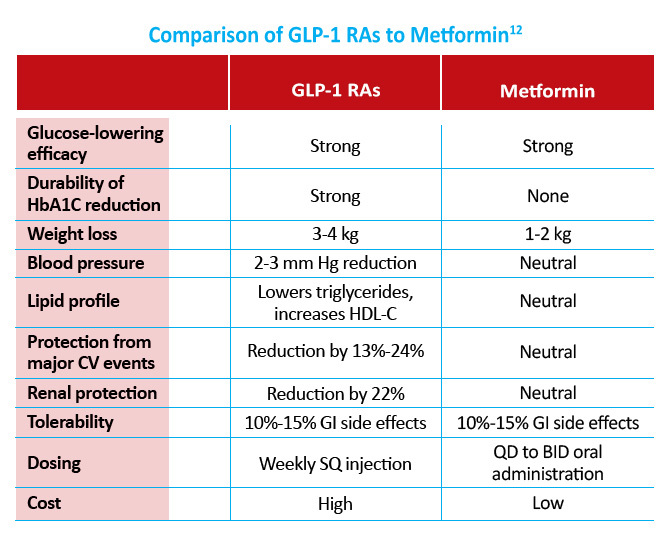
The focus of traditional type 2 diabetes management has been solely on glycemic control. However, given the high risk of cardiovascular disease in patients with diabetes, current guidelines for the selection of pharmacologic agents in patients with established ASCVD risk factors recommend the use of either a GLP-1 receptor agonist or SGLT2i with proven CVD benefit as second-line therapy after metformin.7
GLP-1 Receptor Agonists
- In the SUSTAIN-6 study of 3297 patients with type 2 diabetes and high CV risk, semaglutide use was associated with a significant reduction in nonfatal myocardial infarction (2.9% vs 3.9%; HR, 0.74; P=0.12) and nonfatal stroke (1.6% vs 2.7%; HR, 0.61; P=0.04) compared to placebo. Rates of death from cardiovascular causes were similar between the two groups. At baseline, 83% of patients in the study had established cardiovascular disease, chronic kidney disease, or both.8
- In the LEADER trial of 9340 patients with type 2 diabetes and high CV risk, significantly fewer patients who received liraglutide compared to placebo died from CV causes (4.7% vs 6.0%; HR, 0.78; P=0.007). The rate of death from any cause was also significantly lower in the liraglutide group than in the placebo group (8.2% vs 9.6%; HR, 0.85; P=0.02).9
- Several other GLP-1 receptor agonists have failed to show CV benefit in patients with type 2 diabetes. In patients with type 2 diabetes and acute coronary syndrome, the addition of lixisenatide to usual care did not reduce the rate of major CV events (13.4% vs 13.2%) or rate of hospitalization for heart failure (HR, 0.96) compared with placebo.10 The EXSCEL study group found that once-weekly exenatide did not reduce the incidence of major CV events compared to those who received placebo in 14,752 type 2 diabetes patients (11.4% vs 12.2%; HR, 0.91).11
The cardiovascular benefits of GLP-1 receptor agonists outweigh those of metformin. However, current guidelines still recommend metformin as first like therapy in patients with type 2 diabetes.
SGLT2 Inhibitors
- The EMPA-REG study of 7020 patients randomized to either 10 mg or 25 mg empagliflozin or placebo once daily found a significantly lower rate of death from cardiovascular causes (3.7% vs 5.9%), hospitalization from heart failure (2.7% vs 4.1%), and death from any cause (5.7% vs 8.3%) in the empagliflozin group compared to placebo. The primary outcome of death from CV causes, nonfatal MI, or nonfatal stroke occurred in significantly fewer patients in the empagliflozin treatment group (10.5% vs 12.1%; HR, 0.86; P=0.04).13
- The CVD-REAL study compared the risk of cardiovascular death and hospitalization for heart failure in 309,056 type 2 diabetes patients newly initiated on either SGLT2 inhibitors or other glucose-lowering drugs. Use of canagliflozin, dapagliflozin, and empagliflozin was associated with lower rates of hospitalization for HF (HR, 0.61) and death (HR, 0.49) compared to other glucose-lowering drugs.14
- In a systemic review and meta-analysis of the effects of SGLT2 inhibitors on CV events and death, SGLT2 inhibitors protected against the risk of major adverse CV events (RR, 0.84;95% CI, 0.75-0.95; P=0.006), CV death (RR, 0.63; 95% CI, 0.51-0.77; P<0.0001), heart failure (RR, 0.65; 95% CI, 0.50-0.85; P=0.002), and death from any cause (RR, 0.71; 95% CI, 0.61-0.83; P<0.0001). There was an increased risk of nonfatal stroke with SGLT2 inhibitor therapy (RR, 1.30; 95% CI, 1.00-1.68; P=0.049). There was no evidence that any of the seven SGLT2 inhibitors studied differed in CV outcomes or risk of death.15
References
- American Heart Association. Cardiovascular disease and diabetes. Available at www.heart.org/HEARTORG/Conditions/More/Diabetes/WhyDiabetesMatters/Cardiovascular-Disease-Diabetes_UCM_313865_Article.jsp#.WxAZqX-G-Uk
- Kalra S, Mukherjee JJ, Venkataraman S, et al. Hypoglycemia: the neglected complication. Indian J Endocrinol Metab. 2013;17:819-834.
- Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. 2015;314:52-60.
- Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Cardiol. 2017;120(1 suppl):S37-S47.
- Lee AK, Warren B, Lee CJ, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care. 2018;41:104-111.
- Lee AK, Lee CJ, Huang ES, et al. Risk factors for severe hypoglycemia in black and white adults with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2017;40:1661-1667.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2019. Diabetes Care. 2019;42(suppl 1):S90-S102.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228-1239.
- Abdul-Ghani M, DeFronzo RA. Is it time to change the type 2 diabetes treatment paradigm? Yes! GLP-1 RAs should replace metformin in the type 2 diabetes algorithm. Diabetes Care. 2017;40:1121-1127.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs. Circulation. 2017;136(3):249-259.
- Wu JH, Foote C, Blomster J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4(5):411-419.

